Systematic Literature
review made simple
The platform allows you to quickly and transparently conduct a systematic literature review: from importing sources and screening to generating reports ready for submission. No manual processing, no loss of information, no unnecessary compromises.
Review headwinds
With the trend towards evidence-based regulatory compliance clear, workloads across the industry have sky-rocketed and show now signs of slowing down
Features that save time
and reduce the risk of errors
The Literature module covers all stages of a systematic review — from importing sources to the finished report. You get full control over the process without unnecessary technical actions
Intelligent duplicate removal
The system automatically detects duplicates by DOI, PMID, and titles and offers manual validation if necessary.
Automated literature search
Connection to major databases (PubMed, Embase, ClinicalTrials, etc.) without switching between tabs.
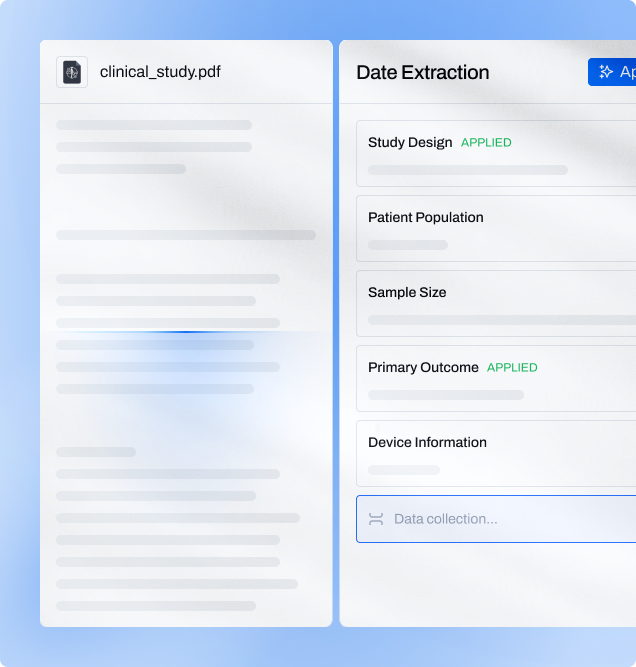
AI highlighting of important fragments
Instantly emphasize key parts of the text for faster analysis.
Generate reports to meet regulatory requirements
Export in formats for MDR, IVDR, FDA with PRISMA, EndNote, Word, Excel.
Intelligent duplicate removal
The system automatically detects duplicates by DOI, PMID, and titles and offers manual validation if necessary.
Flexible screening and filtering
Work with large volumes of sources in a convenient format – with filters, tags, and statuses.
Team collaboration and decision history
Full transparency of reviews, audits, and actions of each participant – for teams of any size.
Generate reports to meet regulatory requirements
Export in formats for MDR, IVDR, FDA with PRISMA, EndNote, Word, Excel.
With the Literature Module, you don’t have
to manually clean up duplicates, transfer
citations to tables, or search
for a lost PDF in the mail. Everything from
searching to reporting works
in an easy-to-understand scenario,
where your contribution is focused
on expertise, not routine
Evidence Cloud™ is built to meet the standards that matter in regulatory work
A simple, controlled process — from search to report
The Literature module covers the full cycle of a systematic review. All steps are structured, transparent and stored in one environment, ready for submission and audit
- Source import and connection
- Automatic duplicate deletion
- Screening and filtering
- Analysis and highlights
Same process —
different level of control
The platform recreates the familiar workflow, but removes the unnecessary: duplication, manual routine, and disparate tools.
Relevant benefits
for each role in the team
The platform takes into account the context, tasks, and typical difficulties of each user.
Choose your role and see how the Literature module fits into your workflow.

Access to peer-reviewed literature for PMS, PMCF, PSUR reports without manual search.

Centralized repository of sources with tags, statuses, and selection history.

The ability to reuse sources and comments in future reports.

Generate reports in standardized formats ready for submission.
87%
optimization
Average level of duplication
reduction in search results
140+
reviews
Systematic reviews created
through the platform
Thousands of sources. Hundreds of reports. One controlled process
The Literature module is already being used to prepare a large volume of documentation in medtech companies that operate under the highest regulatory requirements
30–50%
acceleration
Saving time on screening and reporting
17
knowledge bases
Knowledge bases supported
by the platform
All modules connected in one platform
Evidence Cloud™
Module Connections
Other modules
that work with Literature
Evidence Cloud™ is not a separate tool, but an interconnected system.
The Literature module integrates with other parts of the platform to build
a full cycle of regulatory documentation