CiteSource –
all teams. One library
Literature drives every Medtech workflow—from regulatory submissions to medical affairs claims. CiteSource brings all your references into one searchable, shareable platform that prevents duplicate purchases and ensures audit-ready documentation. Your teams access the literature they need, when they need it.
Review headwinds
Without centralized reference management, teams
rising costs, and critical gaps in knowledge sharing
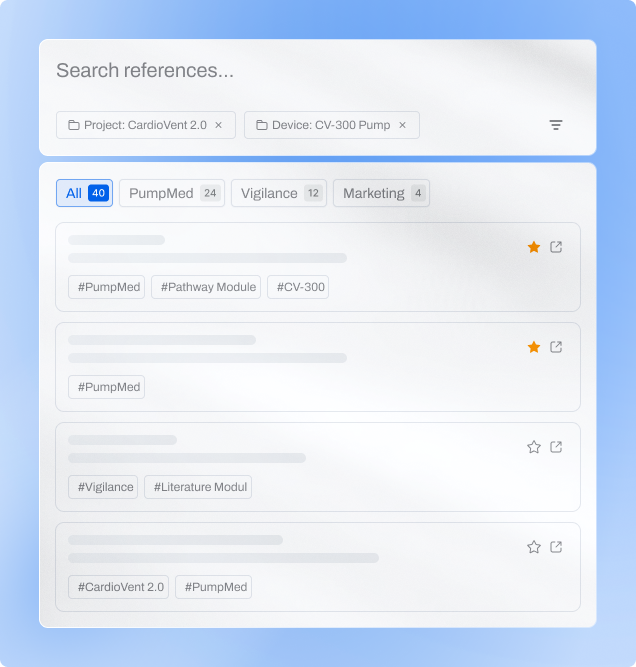
Features built to organize knowledge
and eliminate duplication
CiteSource helps you manage literature across teams — from saving
and tagging sources to tracking their use in submissions. Every reference
stays connected, searchable, and ready for reuse
Intelligent duplicate removal
The system automatically detects duplicates by DOI, PMID, and titles and offers manual validation if necessary.
Track Usage Across Submissions
See exactly where each reference has been used — from CERs to PSURs. Avoid redundant reviews and ensure consistency
Tag and Organize with Ease
Categorize articles by topic, regulation, or internal workflow. Build structured collections for future reuse
Generate reports to meet regulatory requirements
Export in formats for MDR, IVDR, FDA with PRISMA, EndNote, Word, Excel.
Buy Once, Access Forever
Every purchased article lives in your permanent, searchable library. Eliminate 60-80% of duplicate literature costs
Collaborate Without Silos
Share curated literature sets across departments or with external partners. Everyone stays aligned without duplicating effort
Intelligent duplicate removal
The system automatically detects duplicates by DOI, PMID, and titles and offers manual validation if necessary.
Find Any Reference Instantly
AI-powered search understands medical terminology and suggests relevant content. Reduce literature search time
Streamlined Request Management
Complete request tracking and automated notifications. 70% faster literature fulfillment process
Generate reports to meet regulatory requirements
Export in formats for MDR, IVDR, FDA with PRISMA, EndNote, Word, Excel.
A clear path from request to reuse
CiteSource brings structure to how literature is requested, stored, and reused across submissions. Every step is transparent, trackable, and aligned with regulatory workflows — from first request to final report
- Add References
- Organize & Collaborate
- Request & Fulfill
- Export & Share
Fits Your Current Workflow
EndNote, Zotero, and Mendeley backward compatibility out of the box.
Import existing libraries and export references freely between
all major reference managers. No disruption to established processes.
500+
systematic reviews
Completed for audits, submissions,
and PMS
70%
faster fulfillment
Quicker access to requested literature
Trusted by medical equipment manufacturers
Backed by measurable results across regulatory, clinical, and post-market workflows — from faster fulfillment to complete compliance
60–80%
cost reduction
Duplicate purchases avoided
across departments
100%
regulatory compliance
Meets FDA and EU MDR standards
All modules connected in one platform
Evidence Cloud™
Module Connections
Other modules
that work with CiteSource
Evidence Cloud™ is not a separate tool, but an interconnected system.
The CiteSource module integrates with other parts of the platform to build
a full cycle of regulatory documentation




