Pathway – regulatory intelligence
you can use
Get instant alerts when new regulations are published globally, then use AI-powered analysis to understand exactly what they mean for your specific devices. No more spending days deciphering if a 100-page guidance applies to you. Pathway delivers clarity in minutes, not weeks.
Review headwinds
As global regulations evolve at an accelerating pace, tracking and interpreting
relevant updates across regions is becoming unsustainable for most MedTech teams
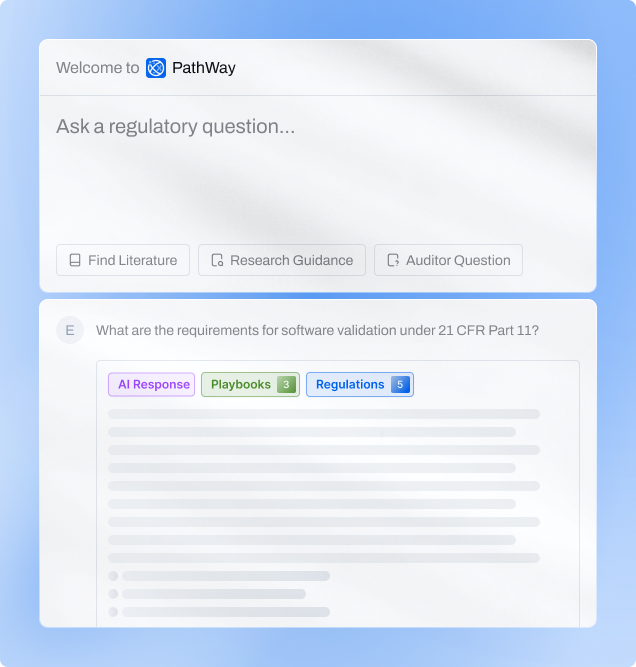
Intelligence Built from Your Evidence
Pathway simplifies regulatory monitoring and interpretation — from filtering global
updates to delivering clear, actionable insights for your team
Intelligent duplicate removal
The system automatically detects duplicates by DOI, PMID, and titles and offers manual validation if necessary.
Map Regulatory Changes Over Time
Track how specific regulations have evolved. See the full context behind shifts in requirements and adapt with confidence.
Stay Aligned Across Teams
Ensure R&D, Regulatory, and QA teams follow the same guidance. Everyone works from the same source of truth.
Generate reports to meet regulatory requirements
Export in formats for MDR, IVDR, FDA with PRISMA, EndNote, Word, Excel.
Never Miss Critical Updates
Scan 50+ global sources and get alerts within hours. Only see what’s relevant to your markets and device class.
Reduce Duplicate Research
All past assessments and decisions are stored centrally. Avoid repeating reviews already completed by other teams.
Intelligent duplicate removal
The system automatically detects duplicates by DOI, PMID, and titles and offers manual validation if necessary.
Understand Impact Instantly
Receive plain-English interpretations and summaries. Know what to do, by when, without guesswork.
Navigate Complex Requirements
Use interactive roadmaps to plan your submissions. Get tailored guidance with clear timelines and next steps.
Generate reports to meet regulatory requirements
Export in formats for MDR, IVDR, FDA with PRISMA, EndNote, Word, Excel.
Insights to Action
Pathway structures how you receive, interpret, and act on global regulatory updates. Every step is targeted, explained, and trackable — so you never miss what matters.
- Configure Your Profile
- Receive Targeted Intelligence
- Access Deep Analysis
- Execute With Confidence
Fits Your Regulatory Workflow
Pathway integrates with your quality systems and document control platforms.
Updates flow directly into your compliance calendar. Export summaries to your QMS.
Link regulatory requirements to your technical documentation.
50+
regulatory bodies monitored
Global coverage of agencies ensures
region-specific compliance.
97%
relevance accuracy
AI surfaces only what applies
to your products and markets.
Comprehensive coverage across global regulations
Built for global compliance teams managing device registrations, submissions, and market access. Pathway ensures no update is missed — with automated monitoring, expert interpretation, and measurable results.
10,000+
regulations indexed
Instant access to current and historical
regulatory data.
40
hours saved monthly
Per user, by eliminating manual tracking
and filtering.
All modules connected in one platform
Evidence Cloud™
Module Connections
Other modules
that work with Pathway
Evidence Cloud™ is not a separate tool, but an interconnected system.
The CiteSource module integrates with other parts of the platform to build
a full cycle of regulatory documentation




